Can ‘forever’ chemicals become less so? This senior thesis works toward smarter cleanup of PFAS.
The class of chemicals known as PFAS — used in firefighting foams, some nonstick cookware, and many other products — can resist heat and repel water. Their chemical bonds are hard to break, and they persist in water sources for decades.
Exposure to them has been associated with cancers, “impacts to the liver and heart, and immune and developmental damage to infants and children,” according to the Environmental Protection Agency, which recently set national limits for PFAS in drinking water.
For her thesis research, Princeton senior Amélie Lemay has crafted computer simulations that could one day help lead the way to removing PFAS pollution from the environment.
Lemay, a civil and environmental engineering major, used simulations to investigate how seven types of molecules behave above bodies of water, where the water meets the air. She modeled their tendencies to mix with water or stick to the water-air boundary, and probed how mixtures of PFAS molecules interact — mimicking the messy reality of contaminated water.
Detailed knowledge of this chemistry could be key to understanding how remediation methods will work in settings like water treatment plants. Over the next few years, utilities across the United States will need to find effective ways to remove PFAS (per- and polyfluoroalkyl substances) from drinking water to comply with the EPA limits.
“Most of our drinking water treatment plants are not set up to deal with these compounds,” said Lemay. “This type of research can eventually lead to better ways to be able to take PFAS out of water.”
Lemay, of Wynnewood, Pennsylvania, came to Princeton with aspirations of using engineering to address environmental challenges. But using computer simulations to understand pollution was not part of her plan.
The summer after her first year, in 2021, Lemay secured internship support from Princeton’s High Meadows Environmental Institute to conduct field work with associate professor Ian Bourg on how rocks weather in the Princeton area and in the French Alps — research with implications for soil nutrients and atmospheric CO2 forecasting.
But COVID-19 travel restrictions were still in place that summer, so Bourg worked remotely with Lemay and several other students to set up simulations exploring the behavior of pollutants ranging from PFAS to anti-inflammatory drugs to insecticides.
“I actually ended up really liking this alternative project, and I think it’s even better suited for me than the original project would have been,” said Lemay, who earned certificates in statistics and machine learning and sustainable energy.


The research was an excellent opportunity for Lemay to build her computer coding skills and learn the intricacies of molecular dynamics simulation software.
“When I first started with Professor Bourg, he had to walk me through step by step how to create a file” simulating a single chemical compound, Lemay said. Over time, she learned to add more complexity, accounting for variables like salinity and surface tension. Now the work is “like second nature.”
The summer project was a new direction in the lab’s research. Bourg, an associate professor of civil and environmental engineering and the High Meadows Environmental Institute, said he was learning along with the students. He quickly realized that he could rely on Lemay: “She’s been thinking like a grad student since the very beginning, in terms of being super conscientious and questioning the way we do things,” said Bourg.
Lemay and Ethan Sontarp, a geosciences major, continued the project as research assistants in Bourg’s group for the next two years. Eventually, they modeled the behavior of more than 80 organic pollutants at the water-air interface.
Lemay and Sontarp were co-first authors of a 2023 paper reporting the results in the journal Environmental Science and Technology. The article has been downloaded more than 2,000 times and is Bourg’s most-read research paper from last year — a testament to its value as a resource for researchers looking to improve the tracking and remediation of pollutants, said Bourg.
In her junior year, Lemay conducted independent work with Professor Barry Rand, who studies the properties of new materials for solar cells, analyzing factors that influence the adoption of rooftop solar energy. She published this analysis last year in the journal Energy Policy.
For her senior thesis with Bourg, she developed complex simulations of how multiple PFAS molecules move and interact at the interface of water and air. Her results have revealed that the contaminants’ movements are not limited only by physical space but also by complex charge interactions among neighboring PFAS molecules.
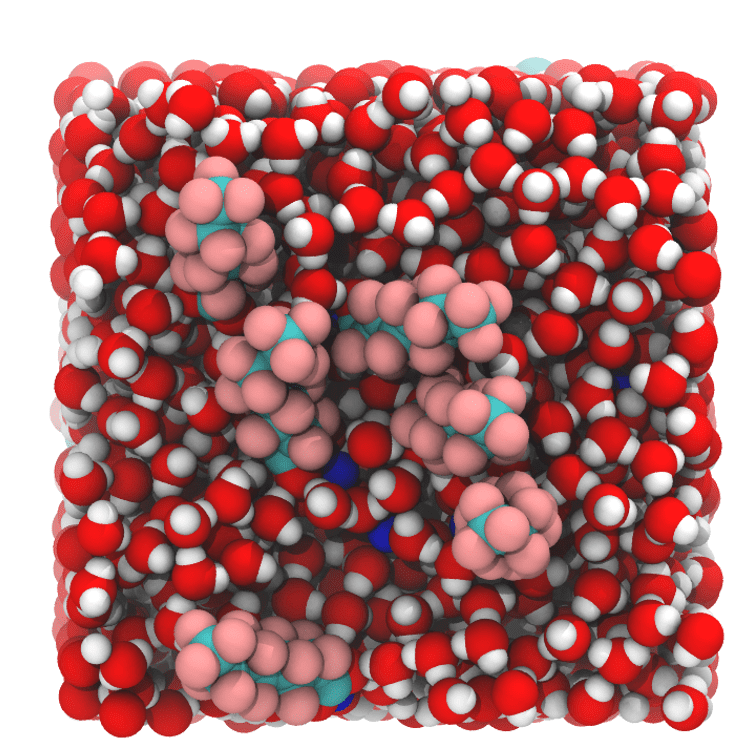

Image by Amélie Lemay
Lemay is now submitting this work for scientific publication. The simulations are a powerful way to understand how pollutants move in the environment, potentially helping to explain how rain interacts with contaminants, and why sea spray and lake spray aerosols are an important source of PFAS exposure in coastal communities. Lemay hopes this understanding can inform strategies to clean up PFAS pollution.
Lemay turned to engineering in high school, when she took part in a summer research program on biomolecular engineering. “In science, you’re seeking to uncover the unknown, which is very important,” she said. “But I found that the problem-solving and design aspects of engineering really appealed to me. I loved how practical and pragmatic the applications were.”
After nearly three years of research at Princeton, Lemay has gained comfort with the uncertainties of the process. “If you pursue something, and you don’t fully understand what the data are showing you at first — that used to be distressing to me,” she said. “But I’ve come to realize that it’s part of the process. You’re trying to do something that’s never been done before. No one has the right answer.”
This summer, Lemay will pursue a project advised by Professor Mark Zondlo analyzing electric vehicle use and neighborhood-level air pollution.
In the fall, she will begin a Ph.D. program in civil and environmental engineering at the Massachusetts Institute of Technology. She’s interested in using computational methods to design chemicals for programmed degradation, to prevent problems with environmental contamination in the future.
“I think Princeton’s focus on undergraduate research really sets this institution apart,” said Lemay. “I’m grateful to have had the chance to work with multiple mentors who have shown me … how to design solutions and search for knowledge, and then share that with the greater community.”




